
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Rocks are composed of minerals that have a specific chemical composition. As is discussed in later chapters, there are three types of rocks composed of minerals: igneous (rocks crystallizing from molten material), sedimentary (rocks composed of products of mechanical weathering (sand, gravel, etc.) and chemical weathering (things precipitated from solution), and metamorphic (rocks produced by alteration of other rocks by heat and pressure. Typically, substances like coal, pearl, opal, or obsidian that do not fit the definition of mineral are called mineraloids.Ī rock is a substance that contains one or more minerals or mineraloids. But once that clam shell undergoes burial, diagenesis, or other geological processes, then the calcite is considered a mineral. Because of these discrepancies, the International Mineralogical Association in 1985 amended the definition to: “A mineral is an element or chemical compound that is normally crystalline and that has been formed as a result of geological processes.” This means that the calcite in the shell of a clam is not considered a mineral. Calcite is quite often formed by organic processes, but is considered a mineral because it is widely found and geologically important. Both are considered minerals because they were classified before the room-temperature rule was accepted as part of the definition. For example, water and mercury are liquid at room temperature. Some natural substances technically should not be considered minerals, but are included by exception. In geology, the classic definition of a mineral is: 1) naturally occurring, 2) inorganic, 3) solid at room temperature, 4) regular crystal structure, and 5) defined chemical composition. The term “minerals” as used in nutrition labels and pharmaceutical products is not the same as a mineral in a geological sense. Identify minerals using physical properties and identification tables.List common non-silicate minerals in oxide, sulfide, sulfate, and carbonate groups.Describe the silicon-oxygen tetrahedron and how it forms common silicate minerals.Describe chemical bonding related to minerals.Derive basic atomic information from the Periodic Table of Elements.Describe the basic structure of the atom.The largest crystal found here is 39 feet (12 meters) and 55 tones.Īt the end of this chapter, students should be able to:
These selenite (gypsum) crystals, found in The Cave of the Crystals in Naica, Mexico, has some of the largest minerals ever found. Announcement: Chapter quizzes are not working as of summer 2023.

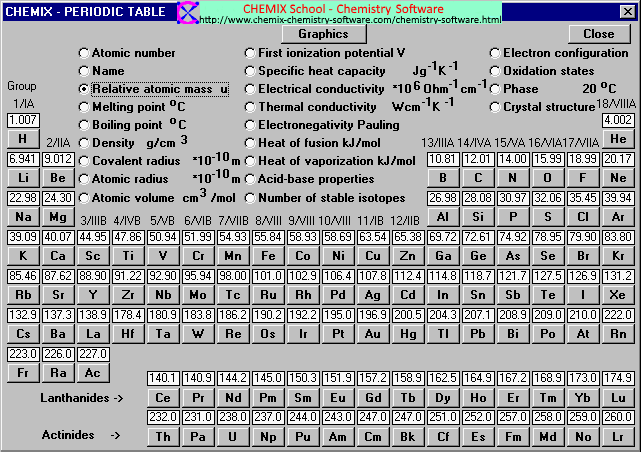
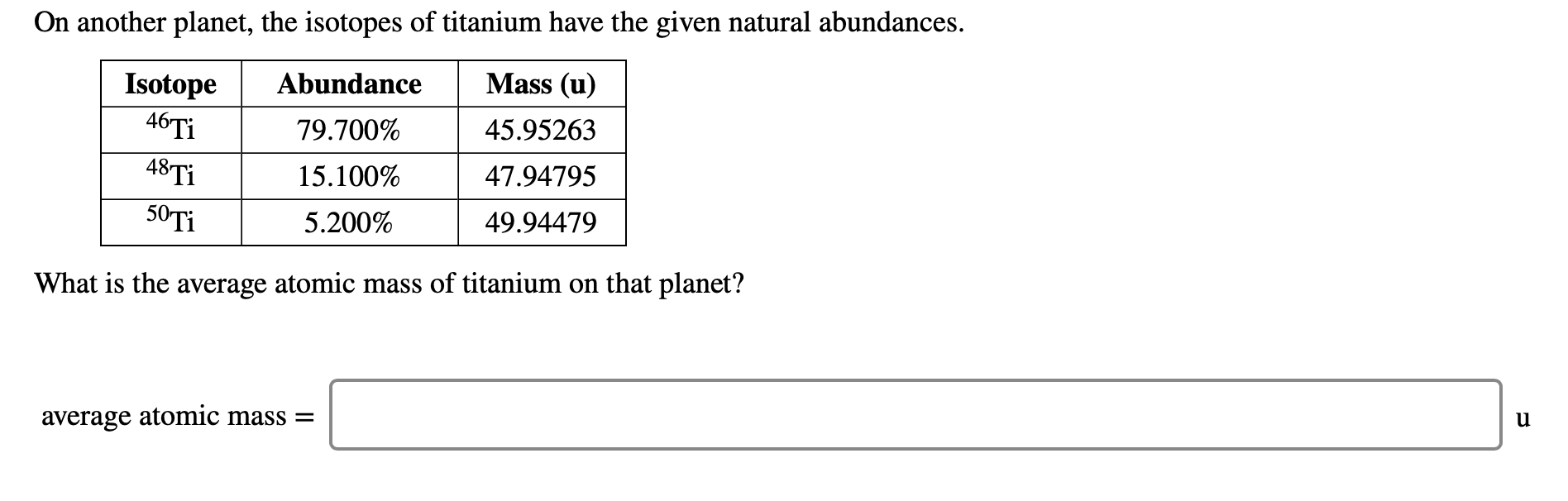
The average atomic mass of titanium will thus beĪvg. The decimal abundances for these five isotopes will be: Simply put, each isotope i will contribute to the average atomic mass of the element in proportion to its decimal abundance, which is simply the percent abundance divided by 100.Ī v e r a g e a t o m i c m a s s = ∑ i m a i × a b u n d a n c e o f i Now, the average atomic mass of titanium is calculated by taking the weighted average of the atomic masses of its stable isotopes. When the problem doesn't provide you with the actual atomic mass of an isotope, m a , you can use its mass number, A, as an approximation of its atomic mass.


 0 kommentar(er)
0 kommentar(er)
